蝙蝠蛾拟青霉-蛹虫草复方的安全性评价及调节免疫活性研究

打开文本图片集
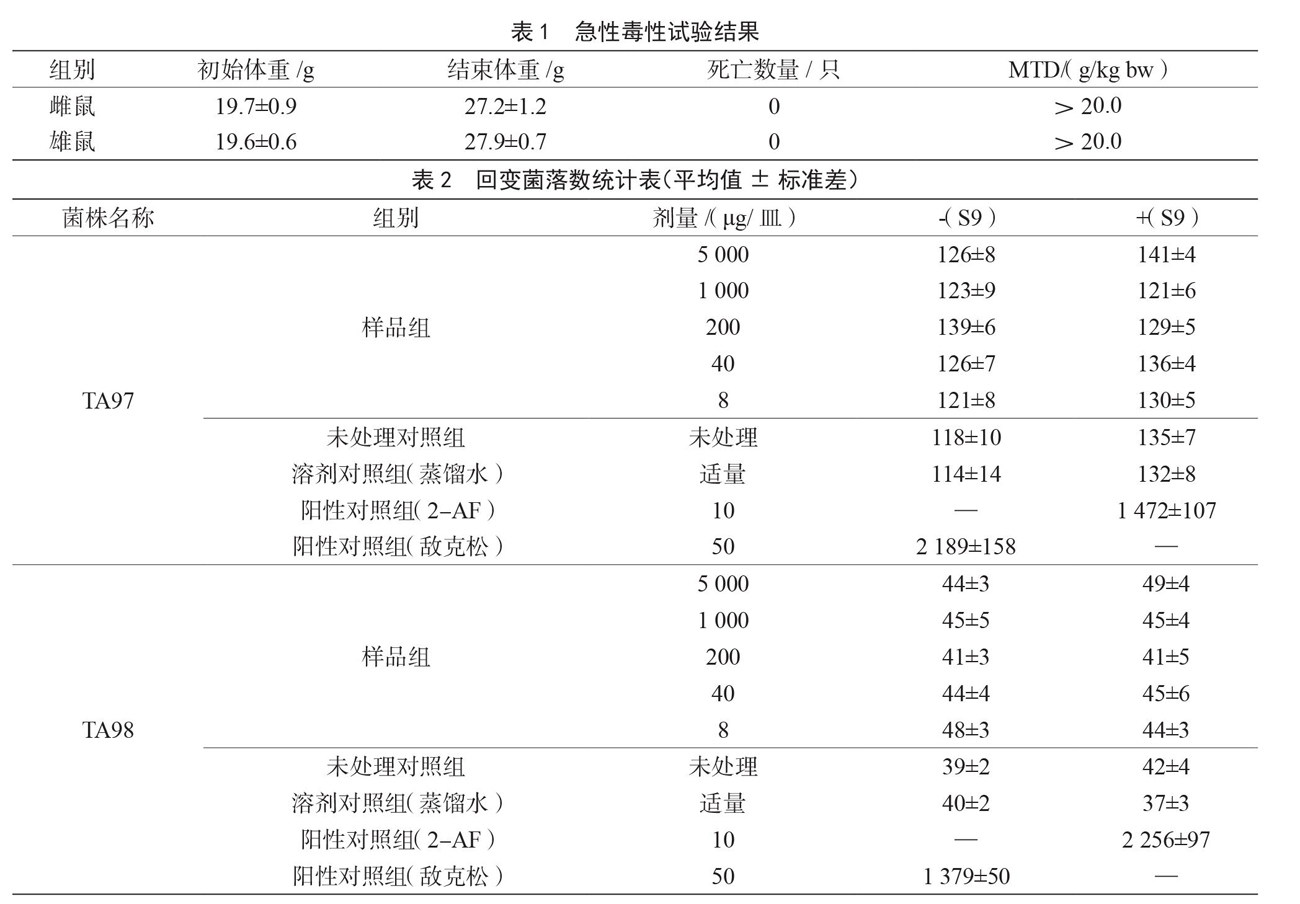
摘 要:目的:评价蝙蝠蛾拟青霉-蛹虫草复方安全性与免疫增强活性。方法:采用小鼠急性毒性试验、骨髓细胞微核试验、精子畸形试验、微粒体酶(Ames)试验、迟发型变态反应试验、血清溶血素滴度检测和脾细胞抗体生成细胞试验,观察受试物的安全性和免疫功能。结果:蝙蝠蛾拟青霉-蛹虫草复方对小鼠急性毒性最大耐受量>20.0 g/kg bw,属无毒级物质,且小鼠骨髓细胞微核试验、精子畸形试验及Ames试验呈阴性;细胞免疫试验中,中剂量(0.33 g/kg bw)、高剂量(0.99 g/kg bw)复方能显著提高小鼠足跖厚度差值(中剂量:p
关键词:蝙蝠蛾拟青霉;蛹虫草;毒理学;调节免疫;安全性评价
Safety Evaluation and Immunomodulatory Activity of a Combination of Paecilomyces hepiali and Cordyceps militaris
JIAO Chunwei1,2, LIANG Huijia2, SUI Jingjing2, CHEN Jiaming2, XIE Yizhen1,2*
(1.Guangdong Yuewei Biotechnology Co., Ltd., Zhaoqing 526000, China; 2.Guangdong Yuewei Edible Fungi Technology Co., Ltd., Guangzhou 510663, China)
Abstract: Objective: To evaluate the safety and immunomodulatory activity of the combination of Paecilomyces hepiali and Cordyceps militaris. Method: Acute toxicity test in mice, bone marrow cell micronucleus test in mice, sperm malformation test in mice, microsomal enzyme(Ames)test, delayed metamorphosis test in mice, serum hemolysin titer detection and spleen cell antibody - producing cell assay were used to observed to determine the toxicity and immune effect. Result: The maximum tolerated oral acute toxicity of the compound in mice was
>20.0 g/kg bw, which was a non-toxic substance, and the mouse bone marrow cell micronucleus test, sperm malformation test and Ames test were negative; in the cellular immunity test, the medium (0.33 g/kg bw) and high
(0.99 g/kg bw) doses of the compound significantly increased the difference in foot and plantar thickness in mice (medium dose: p
Keywords: Paecilomyces hepiali; Cordyceps militaris; toxicology; immunomodulatory; safety evaluation
冬虫夏草(Cordyceps sinensis)是我国传统名贵中药材之一,因其药效显著而被广泛用于中医、藏医及其他民族医学[1-2]。(剩余6226字)