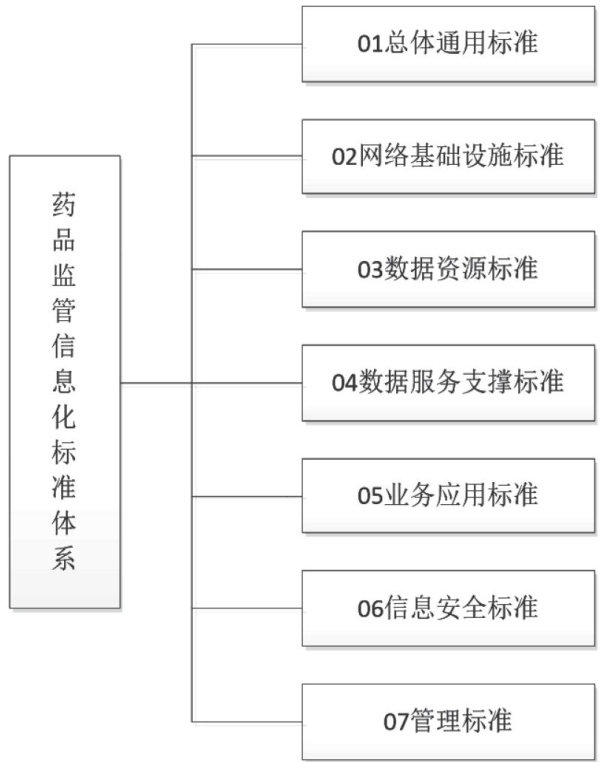
药品数据规范监管的标准化实践

打开文本图片集
Standardized Practice of Regulatory Supervision ofDrug Data
GU Xin1.2 CHEN Lei1*LIU Ying1LU Xi1 GULiping1 QUAN Tongzhen³ QIN Shaohua³ (1.Hubei StandardizationandQuality Institution;2.Hubei Optics Valley Standard Innovation TechnologyCo,Ltd.; 3.HubeiMedical ProductsAdministrationIT&ES Centre)
Abstract:Data qualityis the foundationofpharmaceutical supervision.To acelerate thedevelopmentof intelligent pharmaceutical supervision in Hubei province,fivelocal standards were developed in2O24.Theimplementationof these standardscanefectivelyunifydataformats,qualitystandards,andexchangeprotocols.Thestandardsregulatetheentire processofdrugregulatorydataqualitytoensurethequalityandusabilityofdrugdata,whichprovidesstrongsupportfordrug regulatory data quality management in Hubei.
Keywords:standardization;drugdata;data supervision;normalization
0 引言
数据质量是药品监管工作的基础[1]。(剩余4038字)