多光谱及分子对接技术研究氯霉素与胰蛋白酶相互作用

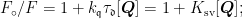
打开文本图片集
中图分类号:0657.3 文献标志码:A 文章编号:1671-4652(2025)01-0070-09
DOI:10.16872/j.cnki.1671-4652.2025.01.009
ABSTRACT:Inorder to explore the interaction mechanismbetween chloramphenicoland trypsin,andto study the influence of chloramphenicolonthe structure and activityof trypsin,this paper studied the interactionbetween chloramphenicoland trypsin under simulated physiologicalconditions by using multispectraland moleculardocking.The results showed thatthefluorescenceintensityof trypsin decreased with the increaseofchloramphenicol concentration.The fluorescence dynamic quenching constants ( Ksv )decreased with the increase of temperature,indicating that chloramphenicol could efectively quenching the fluorescenceof trypsin,and chloramphenicolquenched thefluorescenceof trypsin throughastatic process. The thermodynamic parameters showed that the interaction force between chloramphenicol and trypsin was dominated by the electrostatic atraction model. Circular dichroism(CD)and Fourier transform infrared(FTIR)spectroscopy showed that chloramphenicol changed the secondary structureof trypsin.Enzyme activity studies showed thatchloramphenicolcauseda decrease in trypsin activity in therangeof studied concentrations.Moleculardocking showed that chloramphenicol can form hydrogen bonds withtrypsin HIS-57,TYR-59 and SER-195,respectively. Theresults showed further evidence thatthe presence of chloramphenicol affects the structure of trypsin. In summary,chloramphenicolcan interact withtrypsinandafectthe structure and activityof trypsin.This finding canserve asa solidfoundation for future research into the in vivo interactions between antibiotic medications and digestive enzymes.
KEY WORDS: multispectral technique; molecular docking technique; trypsin; chloramphenicol; interaction
胰蛋白酶(trypsin)是源于动物胰脏中的一种丝氨酸蛋白水解酶,通常由223个氨基酸残基组成,其分子量为23300。(剩余13991字)