基于科学监管的医疗器械检验检测大数据平台研究

打开文本图片集
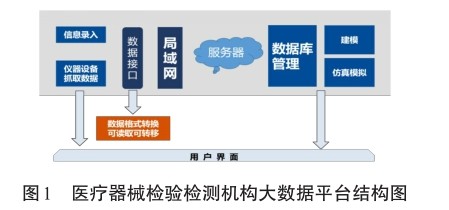
【摘要】本文针对医疗器械检验检测机构数据,设计建立基于科学监管的医疗器械检验检测机构大数据平台,以此有效提高服务客户质量和控制内部质量。此外,建立医疗器械行业大数据平台,将医疗器械生产单位、检验检测机构、使用单位、监管部门的数据实现信息化,用数据驱动医疗器械科学监管,提升服务水平和能力。
【关键词】医疗器械;检验检测;大数据;科学监管
【DOI编码】10.3969/j.issn.1674-4977.2023.05.037
Research on Big Data Platform for Medical Device Inspection and Detection Based on Scientific Supervision
CHEN Xiuwen1, XU Xinrong1, LIU Bin2*, DING Jinju2, LIAN Xiaoqi2
(1.Medical Device Research and Testing Center of South China University of Technology, Guangzhou 537199, China; 2.Dawan Branch Center for Technical Evaluation and Inspection of Medical Devices, National Medical ProductsAdministration, Shenzhen 518000, China)
Abstract: Based on the data of medical device inspection and testing institutions, this paper designs and establishes a Big data platform for medical device inspection and testing institutions based on scientific supervision to effectively improve customer service quality and control internal quality. In addition, establish a Big data platform for the medical device industry to make the data of medical device manufacturers, inspection and testing institutions, users, and regulatory authorities informationized, and use data to drive scientific supervision of medical devices to improve service levels and capabilities.
Key words: medical devices; inspection and testing; big data; scientific regulation
近年来,我国医疗器械产业保持快速发展,成为生命健康领域发展最快的行业。(剩余4395字)