盐酸马尼地平原料药中杂质的结构确证及含量测定

打开文本图片集
中图分类号:TB9 文献标志码:A文章编号:1674-5124(2025)07-0095-09
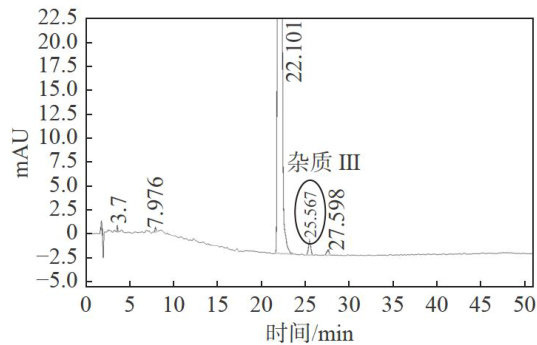
Abstract: Based on the synthetic process route,the impurities in the raw material of Manidipine hydrochloride were separated, confirmed structure and content determination. Agilent ZORBAX SB-C18( 4.6m×250 mm, 5 μm) was used as a column. The detection wavelength was set at 229nm ,the column temperature was 40°C , and the sample size was 20μL .Flow rate was 1mL/min ,mobile phase A was 0.01mol/L ammonium acetate solution,mobile phase B was acetonitrile, gradient elution.The experimental results showed that the byproduct of the synthesis process of Manidipine hydrochloride produced the impurity 5-(3-nitrophenyl)-3-methyl-2-cyclohexene-1-one under acidic conditions, and the impurity dehydromanidipine was produced under light irradiation. The linear relationships of Manidipine hydrochloride impurities were all good (r2⩾0.9997) in the concentration range. The RSDs of precision and solution stability tests were all less than 3.0%(n=6) .The average recoveries of the three impurities ranged from 97.47% to 102.56% ,and the RSDs were all less than 2.0%(n=9) . The study of these impurities is of great significance for the quality control of Manidipine hydrochloride.
Keywords: Manidipine hydrochloride; impurities; structural confirmation; content determination
0 引言
盐酸马尼地平是第三代二氢吡啶类钙离子拮抗剂,能够抑制L型和T型双通道钙离子,高选择抑制血管平滑肌中细胞电压,扩张动脉血管平滑肌,从而降低了血管外周阻力,起到降低血压作用[1-3]。(剩余15324字)