乙二醇-1,2-丁二醇-5-壬酮体系液液平衡数据的测定

打开文本图片集
中图分类号:TQ028.4 文献标志码:A DOI:10.3969/j.issn.1003-9015.2025.01.004
Determination of liquid-liquid equilibrium of the ethylene glycol - 1,2-butanediol- 5- nonanone ternary system
CHEN Peiyu1, WANG Chengxi1, WANG Hengxiu², CHEN Jizhong1 (1.College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310o58,China; 2.Jiangsu HengxingNew Material Technology Co.Ltd.,Yixing 2142OO,China)
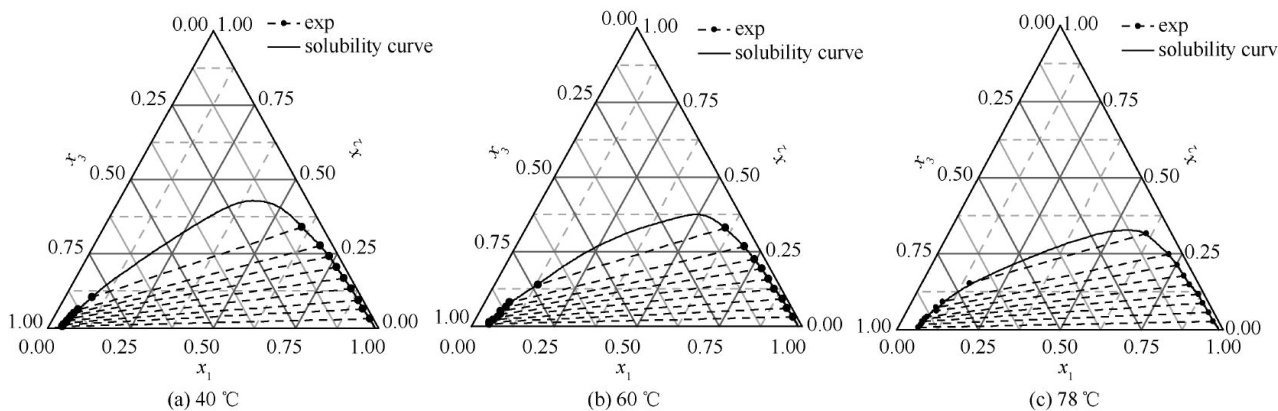
Abstract:Itis diffcult andcostlytoseparate ethylene glycol and1,2-butanediol dueto theirsimilar properties and azeotropy problems.5-nonanone was selected as the extraction agent to separate the mixture of ethylene glycol and 1,2 -butanediol.Liquid-liquid equilibrium(LLE)data(ethyleneglycol-1,2-butanediol- 5-nonanone)wereobserved at 40,60 and 78 C under atmospheric pressure.The Hand equation and the Othmer-Tobias equation were used to examine the consistency of the experimental data,and the NRTLand UNIQUAC model were used to correlate the observed LLEdata,and their model parameters were obtained.RMSD show that the model equations have good applicability in the experimental range.The separation factors and extraction rates of 1,2 -butanediol were also calculated,which show 5-nonanone have a good extraction selectivity and high extraction rate for 1,2 -butanediol at 78 eC. Research results provide theoretical basisand data support for calculation simulation and industrial device design.
Key words: Ethylene glycol; 1,2-butanediol; 5-nonanone; liquid-liquid equilibrium; extraction
1前言
乙二醇(沸点为 197.3°C )是重要的化学溶剂和化工中间产物,主要用于聚酯纤维、防冻液、表面活性剂的制备[1]。(剩余6228字)