达妥昔单抗β治疗神经母细胞瘤的安全性分析

打开文本图片集
【中图分类号】R969.3【文献标志码】A【收稿日期】2024-05-06
【Abstract】Objective:To analyze the clinical characteristics of adverse reactions caused by dinutuximab β for the treatment of neuroblastoma(NB)in China and to provide safety evidence for the rational use of dinutuximab β inclinical practice.Methods:Clinical data wereretrospectivelycollected from16 pediatric patients withNBwho had been treated with dinutuximab β at Shanghai Children' sMedicalCenterAfiliatedtoShanghaiJiaoTong UniversityScholofMedicinefromJanuaryO2 toNovember2O23,ndtheadverse reactions caused by dinutuximab β were summarized and analyzed.Results:The male-to-female ratio was 5:3among the 16 children with NB.The retroperitoneum was the main initial site of involvement,accounting for 75% .Thirteen( 81.25% )patients had high-risk NB.The adverse reactions caused by dinutuximab β mainly included decreased hemoglobin,fever,vomiting,and diarrhea.The incidenceofadversereactionswashighestinthefirstcourseoftreatmentandthemediantimeofadversereactionsas2-5ays.Conclu sion:Targeted monitoring should be carried out at an early stage during dinutuximab β administration.Adverse reactions should be detected and managed earlyto ensure the safetyof medication forchildren.
【Key words】dinutuximab β ; neuroblastoma;adverse reaction
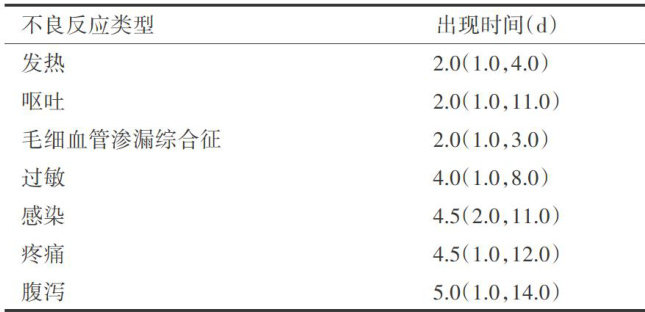
神经母细胞瘤(neuroblastoma,NB)是儿童最常见的颅外实体肿瘤,发病率约占儿童肿瘤性疾病的8%~10% ,病死率约占儿童肿瘤相关性死亡的 12% 215%[1] 。(剩余9490字)