2021—2023年山西省药品监督抽检质量分析

打开文本图片集
'Departmentof Drug Risk Monitoring,Shanxi Provincial Drug SafetyRisk Monitoring Center,Taiyuan 030033, China;²Departmentof Medical Device EvaluationandReview,Shanxi Provincial,Drug Evaluation Center (Shanxi AcademyofMedicine andLifeSciences),TaiyuanO3oo51,China
【Abstract】Objective Through the collection,sorting and statistical analysis of drugquality sampling from 2021 to 2O23,suggestions and references were provided for drug sampling.MethodsThe results of drug quality sampling in Shanxi province from 2O21 to 2O23 were analyzed by descriptive statistics software.ResultsFrom 2021 to 2O23,a total of 8 325 batches were sampled,with a total of 38 batches of non-compliant drugs.The noncompliance rate of sampled drugs decreases from 0.54% to 0.40% . The main category of unqualified sample drugs areChinese herbal medicine slices.And thesampling of non-compliant drugs mainlyoccurred in private hospitals and individual clinics in theusage stage.ConclusionThe overall safety situation of drug qualityin Shanxi provinceis stable and controllable.Thedrugquality isatacomparativelyhighlevel with therateof non-compliant drug decreasing yearbyyear.Drug regulatory authoritiesshould strengthen thesampling inspectionof Chinese herbal medicine slices,increase the sampling proportionat drug usage stage,improve the targeting and accuracyof the drug sampling,ensure the safetyof drug use of the people,and promote the healthyand sustainable development of the pharmaceutical industry in Shanxi province.
【Key Words】Drug quality control; Substandard drugs;Product surveillance, postmarketing DOI:10.19522/j.cnki.1671-5098.2025.04.014
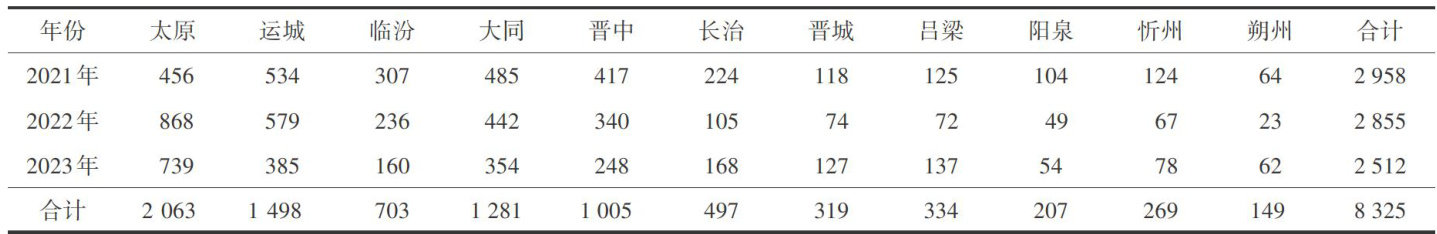
药品一词的由来与中医药学和历史文化的发展密切相关。(剩余4558字)