六边形 Zn 纳米片电催化 CO₂ 制备合成气

打开文本图片集
文章编号:2096-2983(2025)05-0001-12
引文格式:,.六边形 Zn 纳米片电催化 CO2 制备合成气[J].有色金属材料与工程,2025,46(5):1-12. DOI: 10.13258/j.cnki.nmme.20250408001. ZOU Ruyu, TANG Zhihong. Electrocatalytic CO2 reduction to syngas over hexagonal Zn nanosheets[J]. NonferrousMetalMaterials and Engineering,2025,46(5):1-12.
关键词:六边形 Zn 纳米片;自支撑电极;电催化 CO2 还原反应;合成气中图分类号:TQ15 文献标志码:A
Electrocatalytic CO2 reduction to syngas over hexagonal Zn nanosheets
ZOU Ruyu, TANG Zhihong (School ofMaterials and Chemistry, Universityof ShanghaiforScience and Technology,Shanghai 2o93,China)
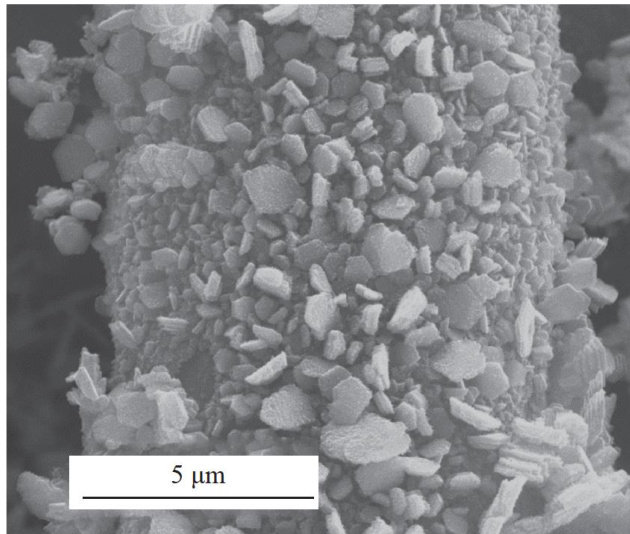
Abstract: Syngas (CO+H2) , is the main raw material for Fischer-Tropsch synthesis, but its traditional preparation method is highly dependent on fossil energy,resulting in a large amount of greenhouse gas emissions. The use of renewable energy to drive the electrocatalytic reduction of CO2 to prepare syngas provides an effective way to achieve the goal of carbon neutrality. However, a large number of studies have been dedicated to exploring the influence of different catalysts on reactions, effectively regulating the molar ratio of CO to H2 on a single catalyst surface remains a significant challenge. Self-supporting Zn-based catalysts (R-Zn2/CC) with hexagonal nanosheet structures were successfully prepared on carbon cloth substrates by the method of electrodeposition combined with pre-reduction, and the performance of electrocatalytic CO2 reduction reaction (CO2RR ) was studied. The results show that the catalyst presents a regular hexagonal nanosheet morphology and exhibits a relatively fast electron transfer rate, which is conducive to the activation of CO2 and thereby enhances its catalytic activity. In addition, at a voltage of -0.95V (vs. RHE), the selectivity of syngas on this catalyst exceeds 90% ,and by regulating the applied potential, the molar ratio of CO to H2 can be continuously adjusted between 0.80 and 2.10.
Keywords: hexagonal Zn nanosheets; self-supporting electrodes; electrocatalytic CO2 reduction reaction; syngas
由 CO2 电催化转化的合成气( CO+H2, ,可以作为费托合成反应的主要原料,是实现碳中和能源系统的有效途径[1-4]。(剩余13751字)