帕泊昔布仿制片的制备及体外溶出度的一致性考察

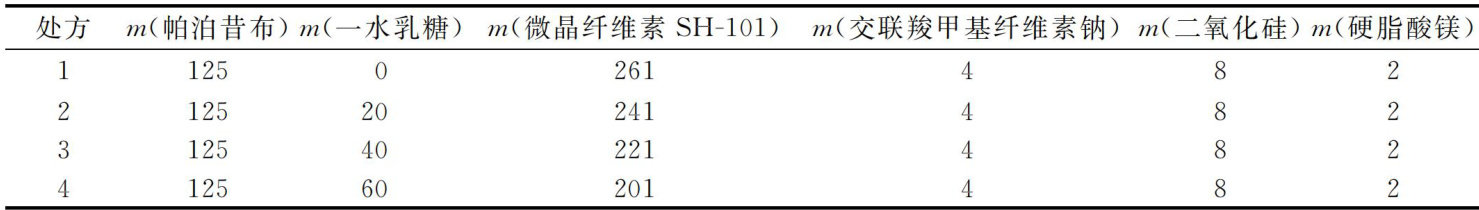
打开文本图片集
中图分类号:R944.4 文献标志码:A 文章编号: 1000-5013(2025)06-0675-10
Abstract:Theoriginal formulation palbociclib tablets was investigatedand consistency in uitro dissolution palbociclib imitation tablets and reference tablets was examined.Referrng to original drug, palbociclib imitation tablets were prepared using direct compresion method.The tablet formulation was determined through single-factor experiments and in vitro dissolution method.An in vitro disolution test was performed using high-performance liquid chromatography(HPLC) to evaluate in vitro dissolution behavior palbociclib imitation tablets.The palbociclibimitation tablets werecompared with reference tablets to evaluate similarity ir dissolution behavior in four different dissolution media.The results showed that homemade palbociclib imitation tablet formulation met relevant requirements for tablet quality specified in pharmacopoeia. The HPLC method for detecting palbociclib in four dissolution media yielded linear correlation coeficients greater than O.99,with objective and accurate measurement results and good recovery rates. Theinvitro dissolution priles palbociclib imitation tablets were similarto those reference tablets infourdissolution media,with f2 values all greater than 50.
Keywords:palbociclib imitation tablets;high-performance liquid chromatography (HPLC);in vitro dissolution;consistency evaluation
乳腺癌是女性高发的恶性肿瘤之一,其病理类型多样,其中,激素受体阳性( HR+) /人表皮生长因子受体2-阴性(HER2-)乳腺癌约占所有乳腺癌病理类型的 70%[1] 。(剩余13313字)