浅谈某院取消头孢菌素类抗菌药物皮肤试验的体会

打开文本图片集
中图分类号: R978.1+1 文献标识码:A 文章编号:1001-8751(2025)04-0284-05
Experience of Canceling Skin Test of Cephalosporins in a Hospital
Du Xiao-hong, Wang Lin, Yao fang (Department of Pharmacy, Inner Mongolia Maternity and Child Health Care Hospital,HohhotInner010020)
Abstract: ObjectiveTo explore the practice and effectivenessof promoting the implementation of the policy of canceling skin tests for cephalosporins.MethodsAnalyze thechanges in pertinent data prior toand following the policy's implementation by looking back at the pertinent data from a hospital's thre-stage cephalosporin skin test and the documented incidence of adverse medication reactions.Stage 1,extract the Guidelines for Skin Tests of Beta-Lactam Antimicrobial Drugs (2O21 Edition) (hereinafter referred toas the Guidelines)issed by the NHSRC. Phase 2,following the issuance of the guidelines to develop our Notice of Cancellation of Routine Skin Tests for Cephalosporins.The third stage,colect medical records of allintravenous useof cephalosporinsafter the hospital formulatedthe Notice on Cancelation of Routine Skin test of cephalosporins,andanalyzed the skintestsituation. ResultsThe skin test rate of cephalosporins in a hospital decreased from 100% before the release of the guidelines to 93.5% before hospital intervention after the release of the guidelines(7 767/8 307), it fell further to 6.7% (766/11 457). Conclusion In addition to the communication and promotion of the Guiding Principles by health administrative departments atallevels,medical institutions have thecourage to take responsibility for the developmentofsystems and norms suitable for implementation in their own hospitals.Atthe same time co-ordination of clinical, pharmacy and nursing and many other disciplines multi-dimensional, multi-channel training and publicity willhelp the implementation of the Guiding Principles, the real cancellation of cephalosporin routine skin test.
Key words: cephalosporins;skintest;scientific management of antimicrobial drugs;analysis;measure
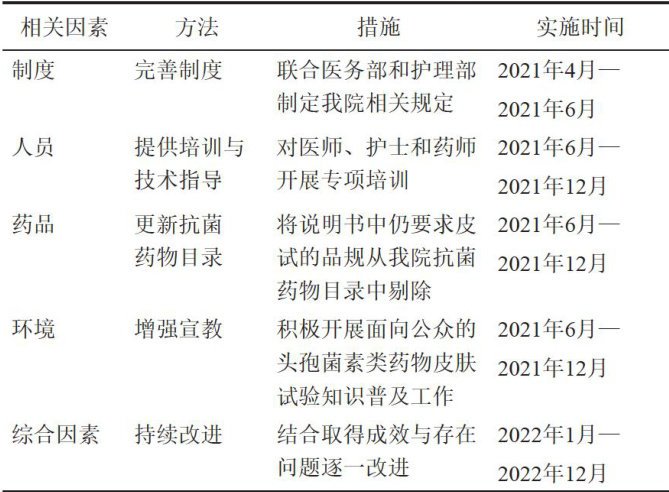
2021年4月13日,国家卫生健康委发布《β-内酰胺类抗菌药物皮肤试验指导原则(2021年版)》[1],指导原则推荐意见:不推荐在使用头孢菌素前常规进行皮试。(剩余6407字)