3-NPBA-TMPDA协同催化合成 N -苄基壬酰胺反应的动力学及机理研究

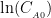
打开文本图片集
关键词:3-硝基苯硼酸: ;N,N,N′,N′ -四甲基丙二胺; N -苄基壬酰胺;反应动力学;反应机理中图分类号:TQ465.92 文献标志码:A DOI:10.3969/j.issn.1003-9015.2025.04.002
Abstract: N -benzylnonanamide and itsanalogues have wide applications invarious fields of medicine,biological control,and tear gas weapons.There are some problems in the synthesis processof these compounds,such as harsh conditions,corrosive and toxic activation reagents.To solve these problems,a novel reaction system was constructedusing nonanoic acid and benzylamineas raw materials,with 3-nitrophenylboronic acid(3-NPBA)and N,N , N′,N′ -tetramethylpropylenediamine (TMPDA)as synergistic catalysts. Aiming to accumulate further knowledge and expertise,the synthesis process and reaction kinetics of N -benzylnonanamide were explored,along with an exploration of the underlying reaction mechanism. The results show that the activation energy of N -benzylnonanamide synthesis catalyzed by the synergistic catalysis of 2.5% 3-NPBA+2.5% TMPDA (molar fraction)is 87.88kJ⋅mol-1 (2号 fitted by first-order reaction kinetics.A possible synthesis pathway for N -benzylnonanamide isproposed as follows: 3-NPBA and TMPDA react with nonanoic acid through dehydration to form a six-membered ring intermediate, which then reacts with benzylamine to form the amide.TMPDA,acting as a Lewis base,donates an electron pair to theboronatomof3-NPBA,stabilizing theintermediate.Meanwhile,duringtheconfiguration transitionof the intermediate,the nitrogenatom of TMPDA forms a hydrogen bond with the hydrogen atom of benzylamine.
Key words:3-nitrophenylboronic acid; N,N,N′,N′ -tetramethylpropanediamine; N -benzylnonanamide;kinetics;reaction mechanism
1前言
N. -苄基壬酰胺及其类似物具有多种生理活性,具有抗菌、缓解炎症性疼痛等作用,普遍存在于天然和人工合成的化学物质中,在医药、生物防治、食品保健和催泪武器等多个领域应用广泛[1-6]。(剩余11360字)