贝伐珠单抗生物类似药与原研生物药的临床转换模式及转换原因分析

打开文本图片集
中图分类号R979.1 文献标志码A 文章编号 1001-0408(2025)18-2297-04
DOI 10.6039/j.issn.1001-0408.2025.18.14
Clinical switching patterns and reasons between bevacizumab biosimilar and originator drugs
OU Min⋅1 ,WANG Yaqin²,ZHU Zhimin³, ZHANG Fangfang4, ZHU Qiongni' (1. Dept. of Pharmacy,Ruijin Hospital,Shanghai Jiao Tong University School of Medicine,Shanghai 200025,China;2.Dept.of Pharmacy, Henan Provincial People's Hospital, Zhengzhou 45003,China;3.Dept.of Pharmacy,Shanghai Eighth People's Hospital,Shanghai 200235,China;4. Dept.of Pharmacy,Qinghai Oilfield Hospital,Gansu Dunhuang 736202, China)
ABSTRACTOBJECTIVEToanalyzeclinical switching paternsandreasons between bevacizumabbiosimilarandoriginator drugs.METHODSThedatawerecollectedfrom1175cancerpatients treatedwithbevacizumabatRuijinHospital,Shanghai Jiao Tong University Scholof Medicinefrom January1,2018,to December31,2023.The patients were divided intooriginatorgroup ( n=250 )and biosimilar group( n=925 ). The switching rate, switching type and reasons of the two groups were compared. RESULTS There were no statistically significant differences intheswitchingrate,switching types,andthe numberof switches between the two groups ( (P>0.05 ). Single,one-way switches were the switching type in both groups. The proportion of patients in thebiosimilar groupwhoswitchedduetoadverseeventswassignificantlyhigherthanoriginator group,whiletheproportioof patients who switched due to treatment costs was significantly lower than originator group ( ΔP<0.05 ).There were no statistically significantdiferences intheproportionsofpatientswhoswitchedduetoeicacyanddrugaccessbilitybetweenthetwogroups 0 ΔP>0.05 ).CONCLUSIONS Theswitching betweenbevacizumab biosimilar and the originator drugs mainly involves single,onewayswitches.Treatmentcostsanddrugacesibilityarethemainfactorsfortheswitchesamong usersoforiginatordrugs,while drug accessibility and adverse events are the main factors for the switches among users of biosimilar.
KEYWORDSbevacizumab;originator drugs;biosimilar;drug switching; safety; adverse reaction;accesbility
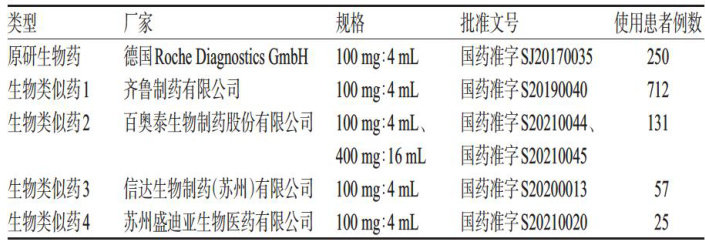
生物类似药是与原研生物药(参考药物)在质量、安全性和有效性上高度相似的生物治疗产品。(剩余7748字)