特殊药品质量安全管理特点及探索新技术、新应用的综合试验场
打开文本图片集
摘 要 通过分析现行法律法规中对特殊药品在供应链各个环节质量安全管理的异同点,提炼出特殊药品的监管特点。契合“十四五”数字化转型的趋势,应用大数据监管、智慧物流、人工智能等信息化技术,探索最新技术、最新应用的特殊药品管理的综合试验场。
关键词 特殊药品 数字化转型 综合试验场
中图分类号:R951 文献标志码:C 文章编号:1006-1533(2022)01-0058-05
The characteristics of quality and safety management of special drugs and the exploration of the latest technology and comprehensive test field of their management
LIU Lu
(Shanghai Municipal Medical Products Administration, Shanghai 200233, China)
ABSTRACT By analyzing the similarities and differences of the current laws and regulations on the quality and safety management of special drugs in each link of the supply chain, the regulatory characteristics of special drugs are extracted. In line with the trend of digital transformation in “the fourteenth Five Year Plan” period, the latest technology and application of the comprehensive test field for the management of special drugs are explored by big data supervision, intelligent logistics, artificial intelligence and other information technology.
KEy wORDS special drug; digital transformation; comprehensive test field
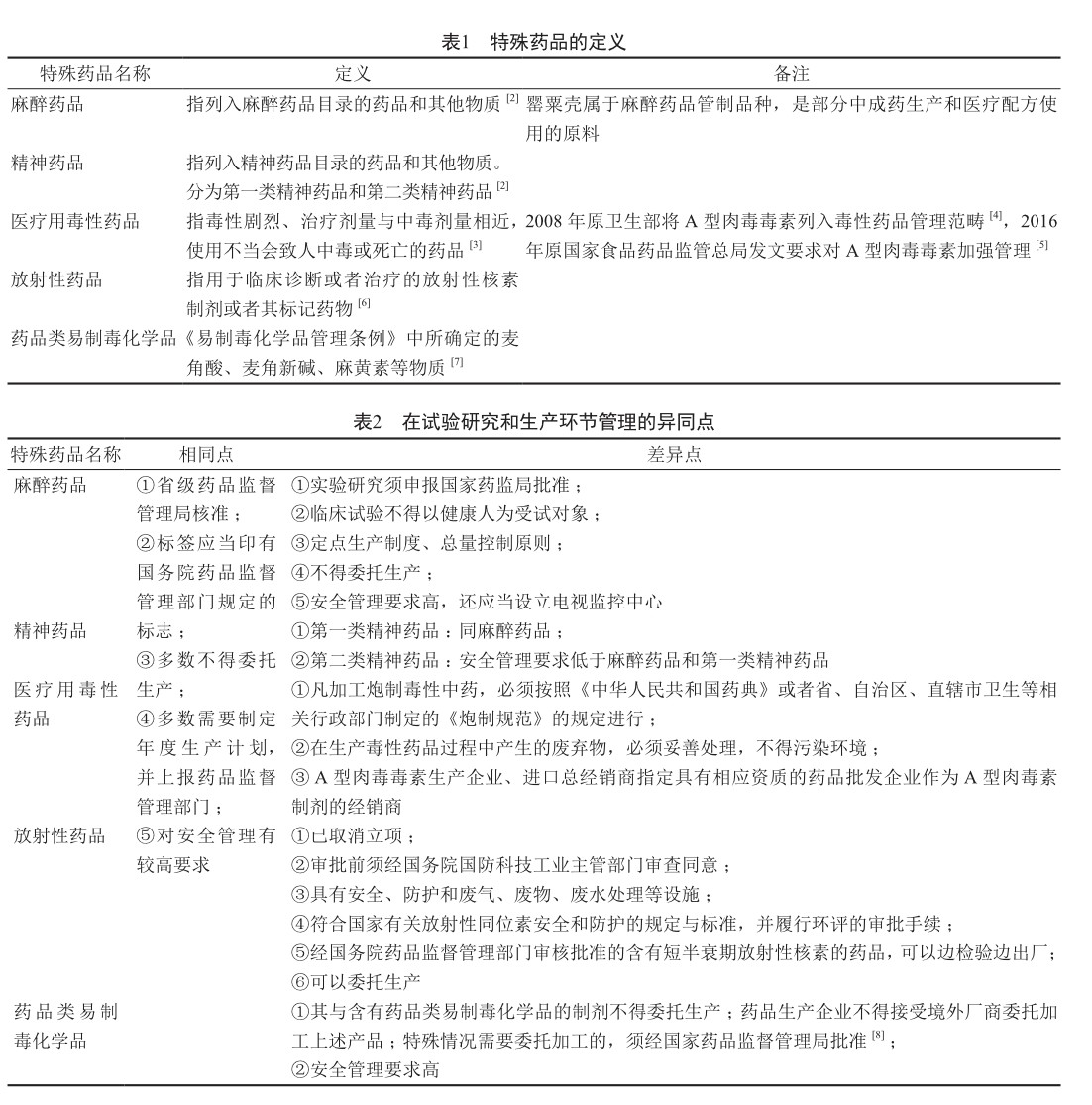
特殊药品管理是药品监管体系的一个重要组成部分,国务院对麻醉药品、精神药品、医疗用毒性药品、放射性药品、药品类易制毒化学品等有特殊管理要求[1]。(剩余4312字)