集采背景下仿制药与原研药临床应用的真实世界研究进展

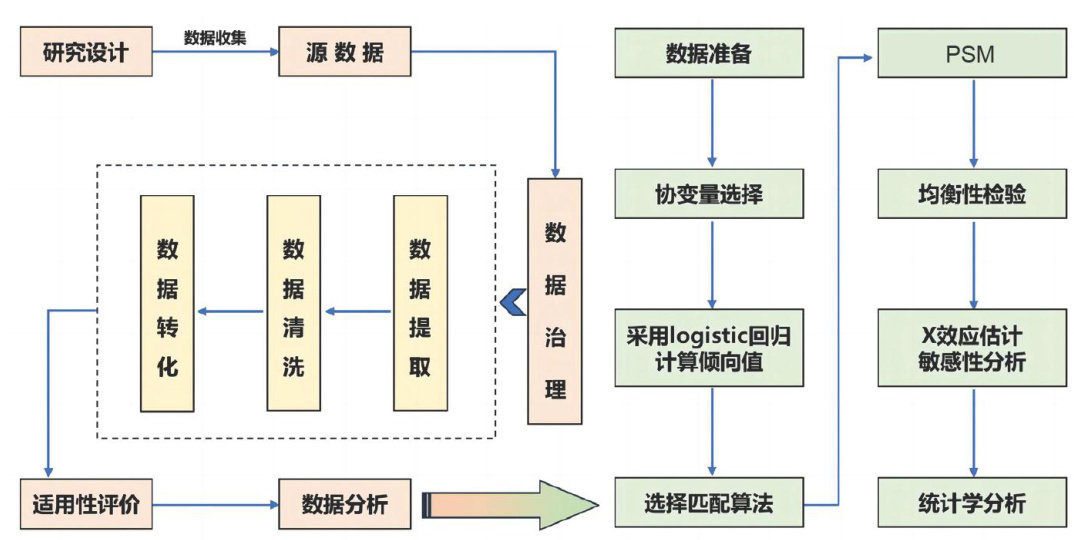
打开文本图片集
【中图分类号】R95 【文献标识码】A
【Abstract】With the normalization and institutionalization of national centralized drug procurement (NCDP) policy,a large number of generic drugs have been included in the clinical frontline, benefiting hundreds of millions of patients. At the same time, real-world studies (RWS)on generics and innovator drugs have been carried out successvely, providing evidence-based support for the promotion and optimization of NCDP policy.This paper systematically reviewed the RWS of generic drugs in the past five years of the NCDP policy,discussed and summarized the evidenceof clinical efcacy,safetyand cost-effectivenessof major generic drugs such as anti-infective drugs, cardiovascular drugs, neuropsychotics, hypoglycemic drugs,and anti-tumor drugs,and analyzed the current RWS status of generic drugs. Overall, the clinical effectiveness and safety of domestic generic drugs are basically the same as that of the innovator drugs, and there is no statistically significant difference,,while generic drugs are more cost-fective. Current research still reveals shortcomings in in data quality and integrity,standardization and rigor of research methods,study coverage and population diversity, pharmacoeconomic evaluation and long-term safety monitoring, which point out the direction forlater research in this field.Through systematic integrationand analysis of the RWS of generic drugs,this review is expected to improve public awareness and recognition of NCDP policy,and provide an important reference for promoting the in-depth implementation of NCDP policy.
【Keywords】National centralized drug procurement; Real world study; Clinical evaluation; Generic drugs; Innovator drugs; Effectiveness; Safety
国家药品集中带量采购(以下简称“集采”)政策自2019年实施以来,截至2025年1月已完成十个批次,覆盖435种药品,药品价格平均降幅达 52% ,部分品种降幅超 95% ,累计节约医疗费用超5000亿元[。(剩余29204字)