瑞波西利联合氟维司群二线治疗绝经后HR+/HER2-晚期转移性乳腺癌的药物经济学评价

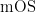
打开文本图片集
【中图分类号】R737.9;R956 【文献标识码】A
【Abstract】 Objective To evaluate the cost-effectiveness of the combination therapy of ribociclib plus fulvestrant compared to fulvestrant monotherapy as a second-line treatment for postmenopausal patients with hormone receptor-positive (HR+) )/human epidermal growth factor receptor 2-negative (HER2-)advanced metastatic breast cancer from the perspective of the Chinese healthcare system. Methods A partitioned survival model was constructed based on MONALEESA-3 study data, with the model cycle of 4 weeks and the time range of 15 years. Total cost, quality-adjusted life years (QALYs) and incremental cost-efectivenessratio (ICER) were compared between ribociclib combined with fulvestrant and fulvestrant monotherapy as a second-line treatment for postmenopausal patients with HR .+ /HER2-advanced metastatic breast cancer. The robustness of the models was validated through one-way sensitivity analysis and probabilistic sensitivity analysis.Results Compared with the fulvestrant monotherapy regimen,the ICER for the ribociclib combined with fulvestrant regimen was 187,958.06 yuan/QALY, which was below the willingness-to-pay (WTP) threshold of three times China’s per capita gross domestic product (GDP) in 2024 (287,247 yuan/QALY).The one-way sensitivityanalysis revealed that the ICER was primarily influenced by the proportion of patients receiving subsequent treatments with the combination therapy or fulvestrant monotherapy,and the utility value of progression-free survival.The probabilistic sensitivity analysis showed that at a WTP threshold of thre times China’s per capita GDP in China in 2024 (287,247 yuan/QALY), the probability of the ribociclib combination therapy being cost-effective was 100% .Conclusion In the context of the Chinese healthcare system, the combination therapy of ribociclib and fulvestrant is more cost-effective than fulvestrant monotherapy for the second-line treatment of postmenopausal patients with HR+ /HER2- advanced metastaticbreast cancer.
【KeyWords】Ribociclib; Fulvestrant; Advanced breast cancer; Postmenopausal; Pharmacoeconomic evaluation; Cost-effectiveness analysis
激素受体 阳性(hormone receptor-positive,HR+ )/人表皮生长因子受体2阴性(humanepidermal growth factorreceptor2-negative,HER2-)的乳腺癌亚型占所有乳腺癌的 65%~75%[1] 。(剩余16263字)