日韩再生医学“双轨制”监管模式介绍及对我国的启示

打开文本图片集
中图分类号R95 文献标志码A 文章编号 1001-0408(2025)15-1832-05
DOI 10.6039/j.issn.1001-0408.2025.15.02
“Dual-track regulatory” models for regenerative medicine in Japan and the Republic of Korea and their implications for China
YANGYifan,XIE Jinping,SHAO Rong(Institute of Regulatory Science for Medical Products,China Pharmaceutical University,Nanjing 211198,China)
ABSTRACTOBJECTIVEToprovidereferencesandrecommendations forimprovingtheregulatoryframeworkforcelland gene therapyproductsandtreatmentsinChina.METHODSThisstudysystematicallexaminedthe“dualtrackregulatory”frameworks forregenerativemedicine productsandtreatmentsinJapanandtheRepublicofKorea,summarized theirbeneficialexperiences, and exploredoptimization strategies for China’sregulatory practices.RESULTS& CONCLUsIONS Both Japanandthe Republic ofKoreahaveestablishedclearmanagementprocesesfortwodistinctpathways“registeredclinicaltrialsforregenerativemedicine products”and“clinicalresearchonegenerativemedicinetreatments”guidedbysharedprinciplesof“riskstraification”and“ful lifecycleoversight".Basedontesefindings,itisecommendedtatChina:strengthentoptierlegislativeframeworktoexplicitly delineatetheregulatoryscope governingcellandgene therapyproductsandtreatments;clarifythe jurisdictionalresponsibilitiesof relevantregulatorybodiestoenhanceoversighteficacy;appropriatelycalibratetheregulatoryscope,andadoptabalanced regulatoryapproachthatharmonizesstandardizationwithinnovationincentives,therebyaceleratingtheclinicaltranslationof regenerative medicine products.
KEYWORDSregenerativemedicine;celland genetherapy;dual-track regulatory;regulatoryscience;Japan;theRepublicof Korea
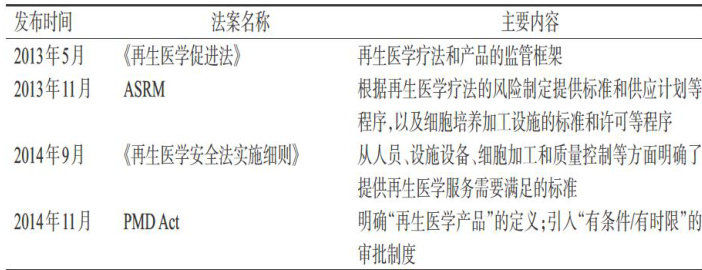
细胞与基因治疗(cellandgenetherapy,CGT)已成为当今全球生物医药产业、生命科技前沿探索最重要的领域之一,其在解决未得到满足的医疗需求方面的潜力引起了社会的广泛关注。(剩余9247字)