HPLC法测定氯地松乳膏中氯霉素含量及其稳定性研究

打开文本图片集
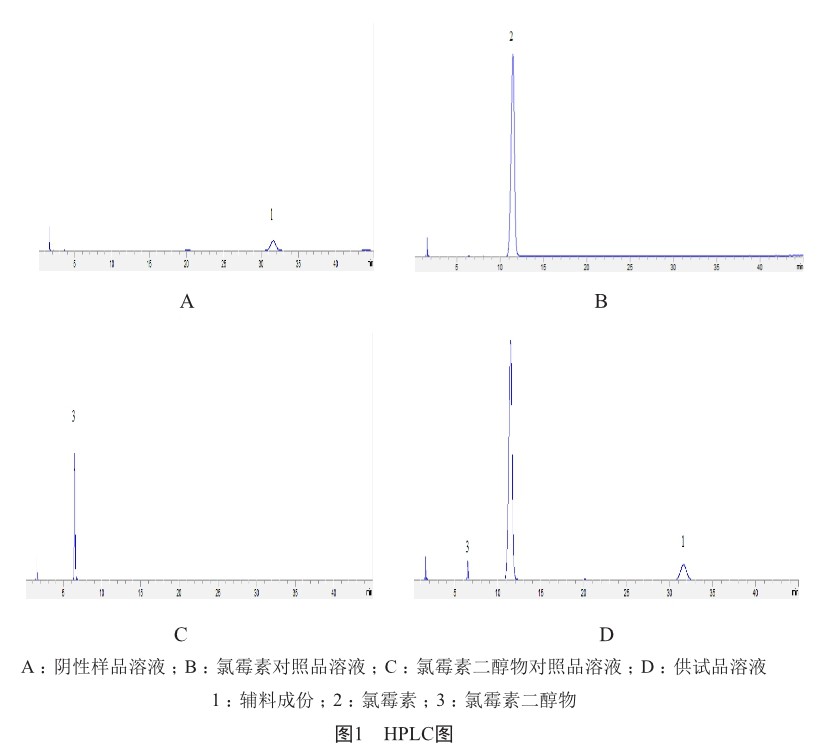
摘 要 本文采用高效液相色谱(HPLC)法测定氯地松乳膏中氯霉素含量,并通过加速试验、长期试验考察制剂稳定性,确保制剂安全、有效。结果发现,氯地松乳膏除遮光、密闭外,在冷处贮存条件下,12个月内性状、含量和微生物限度检查均符合规定,为该制剂的最佳贮存条件和有效期的制定提供了依据。
关键词 氯霉素 氯地松乳膏 高效液相色谱法 含量测定 稳定性
中图分类号:O657.72; R927.2 文献标志码:A 文章编号:1006-1533(2022)19-0066-04
引用本文 赵柳娅, 印杰, 张建中, 等. HPLC法测定氯地松乳膏中氯霉素含量及其稳定性研究[J]. 上海医药, 2022, 43(19): 66-69; 73.
基金项目:上海市临床重点专科建设项目(shslczdzk06504)
Determination of chloramphenicol in chlordiazepoxide cream by HPLC and its stability study
ZHAO Liuya, YIN Jie, ZHANG Jianzhong, WENG Jingyan
(Department of Pharmacy, Zhongshan Hospital affiliated to Fudan University, Shanghai 200032, China)
ABSTRACT The content of chloramphenicol in chlordiazepoxide cream was determined by high performance liquid chromatography (HPLC), and the stability of this formulation was investigated by accelerated and long-term tests to ensure its safety and effectiveness. The results showed that its properties, content and microbiological limits met the requirements within 12 months stored at cold conditions except for shading and confinement, which can provide a basis for its optimal storage conditions and expiry date.
KEY WORDS chloramphenicol; chlordiazepoxide cream; high performance liquid chromatography; content determination; stability
氯地松乳膏为复旦大学附属中山医院自制制剂,以氯霉素和醋酸地塞米松为主药成分,辅料为轻质液状石蜡、甘油、白凡士林、单硬脂酸甘油酯等[1],临床上主要用于神经性皮炎、接触性皮炎、慢性湿疹、局限性瘙痒症等证,效果良好。(剩余5133字)