基于FAERS数据库的瑞加诺生不良事件信号挖掘与分析

打开文本图片集
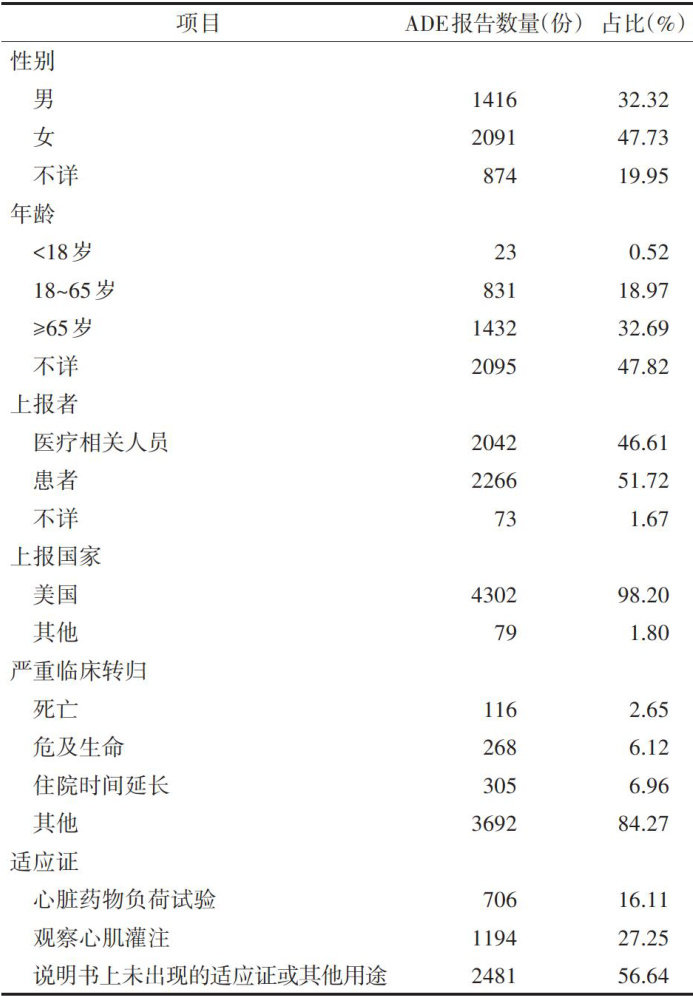
ABSTRACTObjectiveTo mine adverse drug events(ADE) regadenoson based on the Food and Drug AdministrationAdverse EventReporting System(FAERS),andtoprovidescientificevidenceforclinicalaplicationandsafety. MethodsADE reportsassciated with regadenoson from 2008 to 2024 were retrieved from the FAERS database.ADE reports werecodedand classfiedaccording tothesystemorgan classandprefered termthe Dictionary forRegulatory Activities(MedDRA).Thereportingodsratio(ROR)and proportionalreporting ratio wereappliedtoanalyzeADE.ResultsA total4381ADEreportswithregadenoson listedastheprimarysuspecteddrug wereretrieved,including11316ADE occurrences.The reporting population was predominantly female ( 47.73% )and unknown-aged patients ( 47.82% ).The United States was the primary source country ( 98.20% ),and the majority reports were submitted directly bypatients( 51.72% )and medical personnels ( 46.61% ).Regarding ADE outcomes,the proportions life-threatening cases and prolonged hospital stays were relatively high,accounting for 6.96% and 6.12% ,respectively.The detected signals involved 22 system organ class, predominantlyicadiacdiseases,neurologicaldiseasesascularandlymphaticdiseases,astrointestinaldiseasessiatoy system,thoracic and mediastinal diseases,and various examinations.The ADErisk signals were generall consistent with the druginstructions,and2newhigh-risksignalswereadditionalldetected,icludinglossstimulusresponseandpescope (ROR=33.18,15.05).ConclusionDuring theclinicalapplicationregadenoson,inadition toclosely monitoringADE documentedinthedrug’sinstructions,thenewlyidentifiedADEriskshighlightedinthisstudyshouldalsobecareflly monitoredto further enhancethesafetyclinical medication.
KEY WORDSStress echocardiography ;Regadenoson; Pharmacological stress test;Adverse drug event
瑞加诺生作为首个选择性腺苷 A2A 受体激动剂,能够精准扩张冠状动脉,显著提升心肌缺血及冠状动脉微血管疾病的诊断效能[1]。(剩余12296字)