新建药品仓库计算机化系统风险管理与验证

打开文本图片集
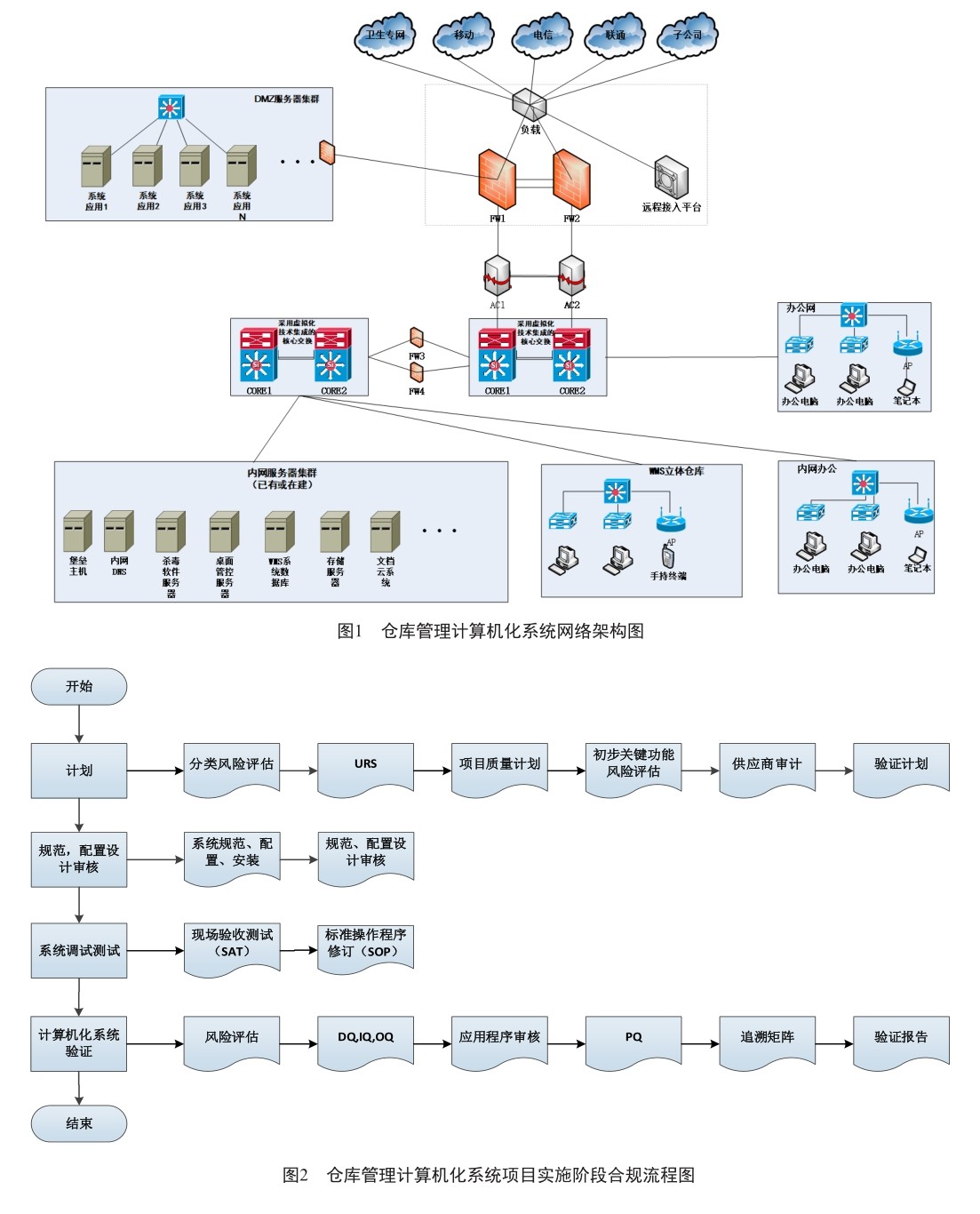
摘 要 《药品生产质量管理规范》附件10计算机化系统是药品生产企业所必需遵循的重要法规之一。本文以新建药品仓库计算机化系统中的管理系统为例,参考了ISPE,PICS及FDA对此类计算机化系统的要求,结合作者经验提出该系统基于风险管理的验证流程。
关键词 风险评估 风险控制 风险回顾 计算机化系统验证
中图分类号:R951 文献标志码:C 文章编号:1006-1533(2023)09-0058-05
引用本文 郑茜, 黄海燕, 严伟民. 新建药品仓库计算机化系统风险管理与验证[J]. 上海医药, 2023, 44(9): 58-62.
Risk management and validation for new drug warehouse management computerized system
ZHENG Xi1, HUANG Haiyan1, YAN Weimin2
[ 1. Sinopharm Geptech (Shanghai) Engineering Co., Ltd., Shanghai 200235, China; 2. School of Pharmacy, Fudan University, Shanghai 201203, China]
ABSTRACT The computerized system in Annex 10 of the Quality Management Code for Drug Production is one of the important laws and regulations that drug manufacturers must follow. The validation process based on the system risk management was proposed by taking the management system in the new drug warehouse computerized systems as an example, referring to the requirements of ISPE, PICS and FDA for such computerized system and combining our practical experience.
KEY WORDS risk assessment; risk control; risk review; computerized system verification
国家药品监督管理局颁布的《药品生产质量管理规范》附件10计算机化系统于2015年12月1日生效执行,法规对制药企业计算机化系统提出了全生命周期风险管理要求,并需根据书面的风险评估结果确定验证和数据完整性控制的程度。(剩余3163字)