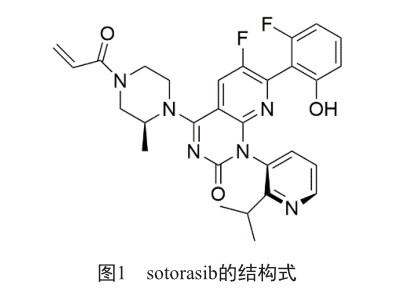
治疗非小细胞肺癌新药:kras基因突变靶向药物sotorasib

打开文本图片集
摘 要 sotorasib是全球首款针对kras基因突变的靶向药物,于2021年5月28日经美国食品药品监督管理局批准上市,用于至少经过一次系统治疗且病情进展的kras基因G12C阳性非小细胞肺癌患者。sotorasib临床疗效较佳,主要不良反应有腹泻、肌肉骨骼疼痛、恶心、疲劳、肝损伤和咳嗽等。
关键词 非小细胞肺癌 sotorasib Kirsten大鼠肉瘤病毒致癌基因同源 靶向药物
中图分类号:R979.19; R734.2 文献标志码:A 文章编号:1006-1533(2022)15-0074-04
引用本文 黄钰雯, 王春晖, 李晓宇, 等. 治疗非小细胞肺癌新药:kras基因突变靶向药物sotorasib[J]. 上海医药, 2022, 43(15): 74-77.
A new drug for treatment of non-small cell lung cancer: sotorasib, targeting kras gene mutations
HUANG Yuwen, WANG Chunhui, LI Xiaoyu, LYU Qianzhou
(Department of Pharmacy, Zhongshan Hospital, Fudan University, Shanghai 200032, China)
ABSTRACT Sotorasib is the first targeted drug targeting kras gene mutations. It was approved by the U.S. Food and Drug Administration on May 28, 2021 for progressed patients with kras G12C positive non-small cell lung cancer after at least oneline systematic therapy. The clinical efficacy of sotorasib is good, and its main adverse reactions include diarrhea, musculoskeletal pain, nausea, fatigue, liver dysfunction and cough.
KEY WORDS non-small cell lung cancer; sotorasib; Kirsten rat sarcoma virus oncogene homolog; targeted drugs
肺癌是世界上最常见的恶性肿瘤, 在我国恶性肿瘤发病率和死亡率均位居第一[1-2]。(剩余7716字)